Density of Nacl Solution
A buffer is a solution that resists changes in its pH when small amounts of strong acid or base is added to itSmall amount is bolded to stress the fact that if you add too much strong acid or base to your buffer its pH will change. Moles solute per kilogram solvent symbol.

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
Q solution m c T where m is the total mass of the resultant solution and c is the specific heat capacity of the resultant solution.
. Pressure of Gas - Measured in Pascal - The pressure of Gas is the force that the gas exerts on the walls of its container. Sodium chloride is an ionic compound with the chemical formula NaCl. Density of Gas - Measured in Kilogram per Meter³ - The Density of Gas is defined as mass per unit volume of a gas under specific conditions of temperature and pressure.
Atomic mass Na 2299 atomic mass Cl 3545 moles of NaCl 3165 g x 1 mol2299 3545 moles of NaCl 3165 g x 1 mol5844 g moles of NaCl 0542 mol kg water density x volume. Sodium chloride is also known as salt. Calculate the molality of the NaCl.
For the XGAANP hybrid η 01 is 32 25 and 24 times that of the single XG solution in NaCl CaCl 2 and MgCl 2. It occurs in oceans and sea waters. Find the mass percent volume percent and massvolume percent of the solute.
Volume percent concentration m number of solute in 100m solution. The density of the resulting solution is considered to be equal to that of water statement holding especially for dilute solutions so the density information is not required. The viscosity of crude oil without water and gas was 234 mPas at 75 C and the density was 093 gcm 3.
Hence there are 3 5 8. Hence there are 3 5844 gms in 1 Litre of water. Vaporpressure data for water are givenin Appendix B b Calculate the mass of propylene glycolC3H8O2 that must be added to 0340 kg of water to reducethe vapor pressure by 288 torr at 40 C.
In its aqueous form it is called a saline solution. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. 3 Molar solution means there are 3 moles of NaCl salt in 1 Liter.
Gram equivalent number of solute in 1L solution. The molecular weight of NaCl is 5844gmol. It is also found as rock salt.
A Calculate the vapor pressure of water above a solutionprepared by adding 225 g of lactose C12H22O11 to 2000 gof water at 338 K. In a solution there is 1110 mL 110605 g solvent and 524 mL 60508 g solute present in a solution. Molality m of NaCl moles of NaClkg water From the periodic table find the atomic masses of the elements.
Weight versus volume percent concentration g number of solute in 100m of solution. Density massvolume Mass of 1 litre of solution 125 gmsml 1000 ml 1 250 gms V volume of water added to make the solution or volume of solvent. Total solution volume is known same equation as case 1.
What is it and how does it work to resist changes in its pH. Table 1 Density and NaOH content of membrane grade caustic soda solutions at 60F 30. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions.
Molecular structure of XG. A 15L solution is composed of 025g NaCl dissolved in water. 3 Molar solution means there are 3 moles of Na Cl salt in 1 Liter.
Calculating the molar enthalpy of neutralisation using the data from the experiment. Since the solutions are mostly water the solutions are assumed to have a density of 10 gmL and a specific heat of 418 JgC. Download high-res image 190KB Download.
I density of each dilute aqueous solution is the same as water 1 g mL-1 at 25C so the mass of solution in grams volume of solution in mL ii heat capacity of each solution is the same as for water C g 418 JC-1 g-1. It is a crystalline solid white. Determine the mole fraction of NaCl in a solution in which 010 moles of the salt is dissolved in 100 grams of water.
Sodium chloride is the salt. With the solution shown in the picture below find the mole percent of substance C. These solutions were stored at room temperature.
Molecular weight of Na Cl 5844. M B C V A 100. Note that with aqueous solutions at room temperature the density of water is approximately 1 kgL so M and m are nearly the same.
This solution was combined with autoclaved stock solutions of other metals to make a 1000 trace metal mixture containing 50 mM FeCl 3 20 mM CaCl2 10 mM each of MnCl 2 and ZnSO 4 and 2 mM each of CoCl 2 CuCl 2 NiCl 2 Na 2 MoO 4 Na 2-SeO 3 and H 3 BO 3 in 60 mM HCl. Expressed as vv when mixture or solute is liquid. OxyChem does not use mercury based electrolytic cells to produce caustic soda.
Expressed as ww wt and for density in many cases. The heat gained by the resultant solution can be calculated using. Molecular weight of NaCl 5844.
Molar Mass - Measured in Kilogram Per Mole - Molar Mass is the mass of a given. The co-products formed from the electrolytic production of caustic soda. Make 2 g100mL of NaCl solution with 1 L water Water properties.
About 1 to 5 of seawater is made of NaCl. OxyChem manufactures caustic soda using either membrane or diaphragm electrolytic cells. 4 4 gms in 1 Litre of water.
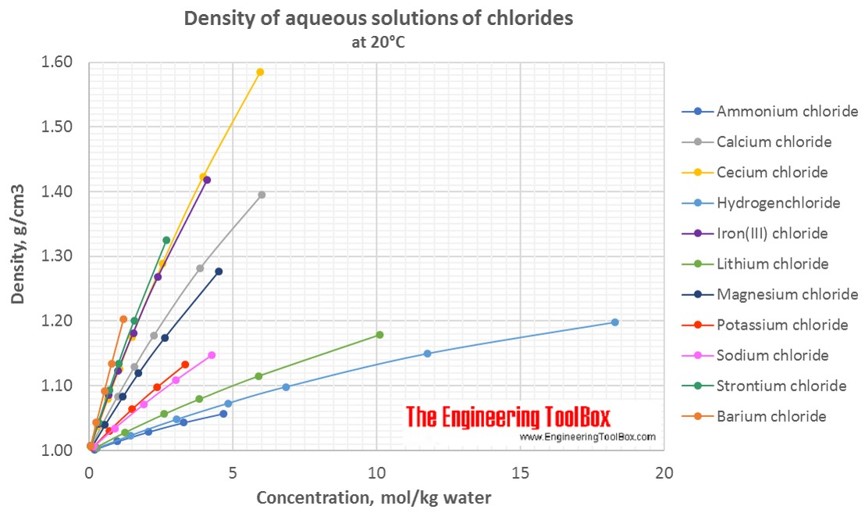
Densities Of Aqueous Solutions Of Inorganic Chlorides

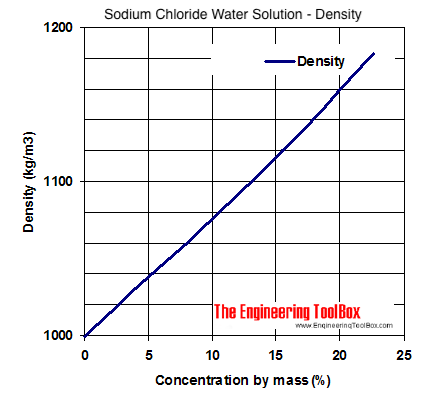
Sodium Chloride Water Solutions
0 Response to "Density of Nacl Solution"
Post a Comment